N. K. Pandey
Sensors and Materials Research Laboratory
Department of Physics, University of Lucknow, Lucknow - 226007, India.
Metal oxide ceramic sensors and transducers have been widely used for the measurement and control of humidity in industrial and household environment. They find broad utility due to the ease with which their compositions and microstructures may be optimized and tailored to specific applications, their stability over large temperature range and low cost. Usually features of ceramics that devices utilize for sensing purposes are the bulk grain phenomena, grain boundary phenomena, or controlled pore structure. Generally, all the three micro structural features come into play, with different relative importance. The feature of the ceramic structure that is utilized in humidity and gas sensing is the porosity. Porosity is normally an undesirable micro structural attribute, since it detracts from many useful properties such as strength, conductivity and chemical stability. In some cases, however, porosity may be desirable, e.g. to reduce the elastic modulus, or as in the present situation, to maximize access of the atmospheric constituents to the large surface area of the semiconductor.
Ceramic humidity sensors can be fabricated from a wide variety of semiconducting oxides. Commercial devices based on porous Al2O3 have been available for a number of years. The latter are capacitive devices that require relatively complex circuitry and frequent recalibration, especially after exposure to wet ambient. A low cost sensor, with a more stable resistivity humidity characteristic was developed in 1978 for use in microwave ovens [1]. Since then it has been manufactured in significant volume. With the fast growth of nanoscience and nanotechnology, the research in metal oxide sensors has developed more accuracy in results.
A comprehensive examination of the characteristics of a wide range of different humidity sensor materials, salt-doped and undoped, resistive and capacitive, reveals that all operate by means of the same physical mechanisms [2]. The situation at low humidity may be understood by referring to Figure 1 In this example A+ metal ions are present in the surface layer of the grains. Because of their small ionic size, high local charge density and strong electrostatic field, they represent very good sites for the chemisorptions of H2O molecules. Upon exposure to the atmosphere, strongly bound water molecules quickly occupy the
sintered in air at temperature of 200 °C-700°C for 3 samples have been exposed to humidity in a specially designed humidity control chamber. Inside the humidity chamber, a thermometer and standard hygrometer are placed for the purpose of calibration. Variation in resistance has been recorded with change in relative humidity. Relative humidity has been measured using the standard hygrometer. Variation in resistance of the pellet has been recorded using a resistance meter. Copper electrode has been used to measure the resistance of the pellet. The resistance of the pellet has been measured normal to the cylindrical surface of the pellet. The electrical resistance at different relative humidity levels of the sensing elements in the form of pellets has been determined by a two-probe method, as the present work is to measure
|
|
available sites. This layer, once formed, is not further affected by exposure to humidity, but it can be thermally desorbed. In undoped sensors, surface cations may still possess high electrostatic charge due to unfilled coordination numbers, and thus present suitable sites that promote adsorption and dissociation of water molecules. Once the first layer has been formed, subsequent layers of water molecules are physically adsorbed. The physisorbed water dissociates due to the high electrostatic fields in the chemisorbed layer:
2H2O =H30+ + OH-
Charge transport occurs when the hydronium ion releases a proton to a neighboring water molecule which accepts it while releasing another proton, and so forth. This is known as the Grotthuss chain reaction and is illustrated in figure 1. It is thought to represent the conduction mechanism in liquid water as well as in the surface layers of humidity sensitive oxides. At high relative humidity (e.g. RH > 40 %) liquid water condenses in the pores as already described, and electrolytic conduction takes place in addition to the protonic transport in the adsorbed layers. Similar mechanisms are believed to be responsible for the properties of capacitive sensors, the principal distinction being the different substrate and pore geometries.
Metal oxide sensing devices suffer from the significant problem of ageing. Aging mechanisms in humidity sensors may be due to one or multiple factors of the following: (1) prolonged exposure of surface to high humidity, (2) adsorption of contaminants preferentially on the cation sites; (3) loss of surface cations due to vaporization, solubility and diffusion, or annealing to a less reactive structure; and (4) migration of cations away from the surface due to thermal diffusion. Generally, the more sensitive a material is to humidity, the more it tends to be susceptible to aging.
Different compositions of metal oxides have been prepared in our laboratory. 10 % weight of glass powder has been added as binder to increase the strength of the sample. The powders have been pressed in pellet shape by uniaxially applying pressure of 200-500 M Pa in hydraulic press machine at room temperature. The samples are having diameter of 4-12 mm and thickness 4 mm. The samples have been.
hours in an electric muffle furnace. After sintering, the the changes in surface conductivity as a function of applied field. The electrical contacts have been made on the surface of pellet by means of two thin copper sheets. Given the high resistivity of the materials under consideration, the potential inaccuracy due to contact resistance is assumed negligible. The experimental sample has been electrically connected to a dc power supply and sinometer in series. After studying humidity sensing properties, sensing elements have been kept in laboratory environment and their humidity sensing characteristics regularly monitored. To see the effect of ageing, the sensing properties of these elements have been examined again in the humidity control chamber after six months. |
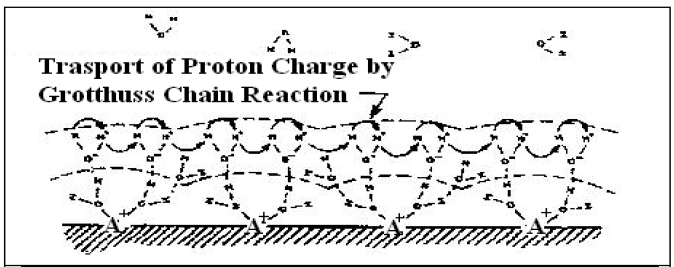
Figure 1 Water adsorption and proton transport on the surface of the humidity sensor
Layer I Underlying layer of water molecules chemisorbed to A= cations This layer is composed of cation-hydroxyl-watercomplexesofintermediatestructure.LayerIIWatermoleculesphysisorbedontolayerI.
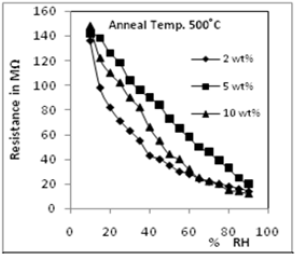
Figar 2 variation in resistence with
humidity.

Figure 4 SEM micrograph of sample with 10 % Cu2O annealed at 500°C
Figure 2 shows one such result with Cu2O doped ZnO. 2, 5 and 10 weight % of Cu2O has been doped in ZnO and characterization and humidity sensing properties of these sensing elements studied Figure 2 shows variation in resistance of the these sensing elements with change in relative humidity. Figure 3 shows the ageing graph and figure 4 the SEM micrograph of the sensing element of 10 % Cu2O doped ZnO. The results obtained with these samples have been very good with good sensitivity, low hysteresis and high repeatability. |
|
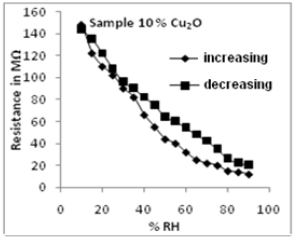
Figar 3 variation in resistence with
humidity for annealing tempeature 500c.
I will be presenting humidity sensing results of these and some other compositions of different metal oxides. I hope my presentation is useful to persons working in the similar field and may result in some collaborative works. 1. Nagamoto S., Nittta T., Kobayashi T. and Nakano M., Proc. Microwave Power Symp. p. 17. Ottawa, June (1978). 2. Nitta T., Terada Z. and Hayakawa S., J. Am. Ceram. Sot. 63, 295 (1980). |
|