Preparation of Mixed Phase Strontium Ferrite Nanoparticles and Effect on Magnetic PropertiesPreparation of Mixed Phase Strontium Ferrite Nanoparticles and Effect on Magnetic Properties Rakesh Kumar Singh1, Amarendra Narayan2, R. N. Roy3 and Avinash C. Pandey4 1 Department of Physics, Patna Women's College, Patna University, Patna, Bihar, India 2Department of Physics, Patna University, Patna, Bihar,India 3 Department of Physics, Samta College, Jandaha, Vaishali, Bihar, India 4Nanotechnology Application Centre, University of Allahabad, Uttar Pradesh, India Abstract Pure phase and mixed phase strontium ferrite powder were prepared using citrate precursor method. The annealing temperature for both samples was 450oC. The samples were structurally characterized using X- Ray diffractometer (XRD) and magnetically characterized using Vibrating sample magnetometer (VSM). The particle size was observed 15 nm and 8nm at same annealing temperature 450oC. Some additional phase appears in addition to hexaferrite. The retentivity and magnetization was found 3.321 emu/g, 36.615 emu/g for pur phase sample while 0.64 emu/g and 33.332 emu/g for mixed phase sample respectively. This behaviour suggests that Key words: Mixed phase ferrite, Citrate precursor method, Magnetic behaviour Introduction Ferrite is an important magnetic material. It has applications in high frequency electronics due to its better heat resistance and higher corrosion resistance [1,2,3]. It has been estimated that in the world market ferrites consist of 40% of the total hard magnetic materials [4,5]. Magnetic core materials and switched mode power supplies are applications involving easy magnetization and demagnetization at high frequencies. They use soft ferrites which have coercivity less than 10 Oe [6]. Materials and Methods Experimental procedure: Nitrates of two cations (Sr2+, and Fe3+) were taken in proper stoichiometric proportions as starting materials for pure phase ferrite. Aqueous solutions of these salts were prepared separately by dissolving the salts in minimal amount of deionized water and stirring constantly. The solutions were then mixed together. Aqueous solution of citric acid was prepared in adequate quantity by weight and was added to the prepared salt solutions. The mixture was heated at Results and Discussion The XRD pattern for SrFe12O19 prepared from a stoichiometric mixture of Ferric Nitrate and Strontium nitrate (sample 1) is shown in figure 2 and peak search analysis data in table 2. The XRD pattern for Strontium nitrate with mixing of phase that was prepared from a SrFe12O19, Fe2O3, SrO2, SrFe2O3 [9]. Although an annealing temperature of 450oC is considered to be on the lower side for 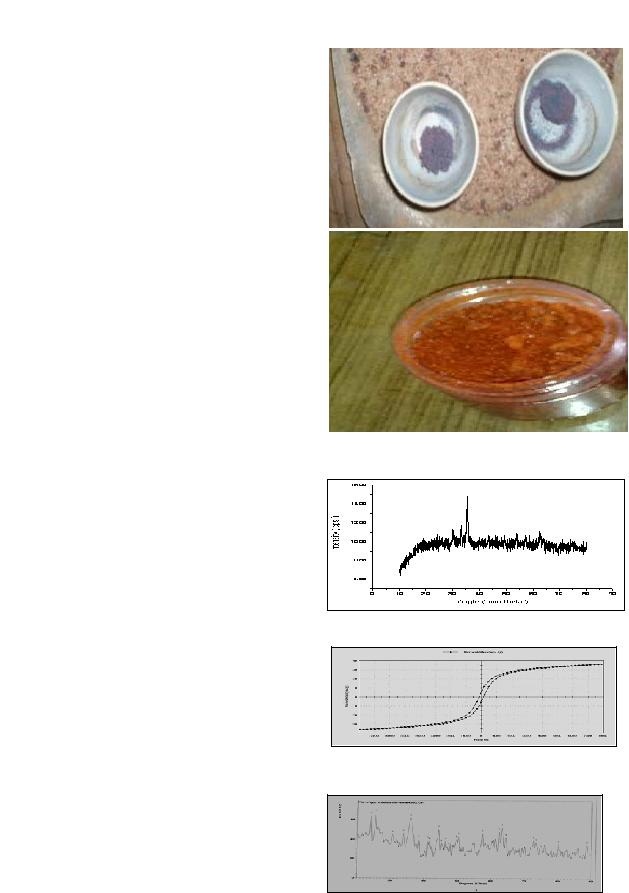
analysis detail for sample 1 is given in table 2 and for sample 2 in table 3 shows interplanner distance(d) intensity of diffraction peaks of the synthesized samples. This results shows that the mechanism for ferrite formation is different for the given two samples. Monophase hexa ferrite become possible at annealing temperatures as low as 700oC in the citrate precursor method where as it requires high temperature of the order 900oC in other chemical method(18). Dspace of the order 3.697 was observed only in sample 2, which belong to hexa ferrite( ICDD file Table 1: Stoichiometric detail for the two ferrite powder samples 
Figure 1: Ferrite annealed powder (above); B. Citrate Precursor for SrFe2O4(below) Figure 2: XRD spectrum for sample 1 Figure 3: Magnetization curve for SrFe12O19 Nanoparticles Figure 4: XRD spectrum for sample 2 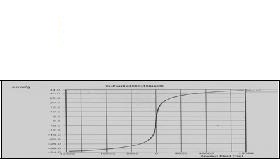
Figure 5: Magnetization curve for sample SrFe2O4 Nanoparticle. The magnetic behaviour was studied using Vibrating sample magnetometer. The magnetic hysteresis loop for sample 1 is shown in figure 3 and for sample 2 is shown in figure 5. A comparison of magnetic parameters is done in Table 4. It can be seen that the impure phase sample (2) shows smaller coercivity and retentivity compared to the pure phase sample, but the magnetization is almost same in both samples. The magnetization tends to saturation around 8000 Oe although there is a slight increasing trend. Looking at the hysteresis curves and magnetic parameter values, the samples do not appear to show superparamagnetism [14]. Several researchers have found the magnetic parameters value of this order in ferrites however they have used different method of synthesis [15,16,17,18]. Retentivity and magnetization are generally reported to be inversely related [19,20]. But we have found in our samples a variation in retentivity that is accompanied with relatively small change in magnetization. The possibility of particle- size dependence of the magnetic properties cannot be ruled out, however. It is also generally considered that high anisotrophy in hexa ferrite has contribution from all the five sublattices. In case of Barium hexa ferrite, prepared using Sodium Citrate Conclusions We have observed small particle size 8nm and 15nm at low annealing temperature 450oC using Citrate precursor method. We have made comparison between pure phase and mixed phase of Strontium based oxide material and found almost similar magnetization values but a relatively large difference in coercivity and retentivity. It is not proper to base any conclusion on just two samples, however it will be interesting to investigate further, the variation of parameters in non- stoichiometric cases as we have done. If a correlation of the type that our data suggest is also observed in other cases, then properties of magnetic oxide materials for given values of magnetic parameters. Acknowledgements The authors are thankful to Professor H. C. Verma, Department of Physics, IIT Kanpur, Dr. R.K. Kotnala, Head, Magnetic standared laboratory, NPL, New Delhi for help in sample characterization. We are also thankful to Nishit K. Pandey, Research Scholar, Department of Physics, Patna University for help in phase analysis of XRD spectrum. References 1.J. Qiu, M. Gu, J. Applied Surface Science, 252 (2005) 2.Ding, J., W.F.Miao, P.G. 'McCormick', R. Street, J. Alloys. Comp. 281(1998) 3.Dobrzanski, L.A., M. Dark, B. Ziebowicz, J. of Achievements in Material and Manufacturing Engineering, 4.Valenzula, R., Magnetic Ceramics, Chemistry of Solid State Materials, 1st ed., Cambridge Univ., USA (1994). 5.Rath, C., Ph.D. thesis, Studies on microstructure dependent magnetic properties of Hametite and 6.Shelling, E.C., Soft Ferrites: properties and applications, 2nd Ed, Butter worth, London, 1988. 7.Carp, O., R. Barjega, E. Segal, M.Brezeanu, Thermochimica Acta, 318 (1998) 8.Dobrzanski, L.A., Fundamental of Materials Science and Physical metallurgy (2006) [in Polish] 9.JCPDS data file NO: 10.Singh R.K., N. Pandey, A. Narayan, MSI Bulletein, Vol.32 11.Singh R.K, A.Yadav, Avinash C. Pandey, R.K.Kotnala, Proceeding, Nat. Conf. Univ. Of Lucknow, (2009) 12.Cullity, B.D., Elements of 13. West, A.R., Solid state chemistry, Wiley Pub. ( 14.Singh R.K, A.Yadav, K. Prasad and A. Narayan, Int. J. Engi .Sci. Tech. No.2, Vol. 2 (2010) 15.Wang, J., Qianwang Chen, Shan Che, J. Magn. Mag. Mater. 280 (2004) 16.Singh R.K, A.Yadav, R.S.Yadav, A.C. Pandey, Manthan, Int. J. Vol. 8 ( 2008) 17.Kim, S.G, 18.Sankaranarayanan ,V.K. and D.C.Khan, J. Magn. Mag. .Mat. 153(1996)
Corresponding Author: rakeshpu@yahoo.co.in |
|||||||||||||||||||||||||||||

