British scientist Sir George Gabriel Stokes first described the phenomenon of fluorescence in 1852. He coined the term when he observed that the mineral fluorspar emitted red light when it was illuminated by ultraviolet excitation. He found that fluorescence emission always occurred at a longer wavelength than that of the excitation light. Early investigations in the 19th century showed that many specimens (including minerals, crystals, resins, crude drugs, butter, chlorophyll, vitamins, and inorganic compounds) fluoresce when irradiated with ultraviolet light. However, it was not until the 1930s that the use of fluorochromes was initiated in biological investigations to stain tissue components, bacteria, and other pathogens.
What is fluorescence?
Fluorescence is the property of some atoms and molecules to absorb light at a particular length and to subsequently emit light of longer wavelength. In other words, if you shine light on some molecules, you may see light of a different color emitted from that molecule. This is known as fluorescence. The molecule absorbs high energy light (blue, for example). This increases the energy of the molecule, represented as the top grey line in the Figure 1 (an "excited" molecule). Some of the energy from the blue photon is lost internally (represented by the red squiggly arrow in the figure 1). The molecule then emits a photon with less energy, green in this example. Fluorescein is a common dye that acts in exactly this way, emitting green light when hit with blue excitation light. The color of light emitted is material dependent, and likewise the excitation light wavelength depends on the material. (There are other forms of inelastic scattering; fluorescence is particularly strong.
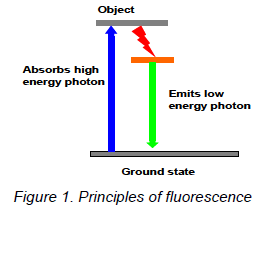
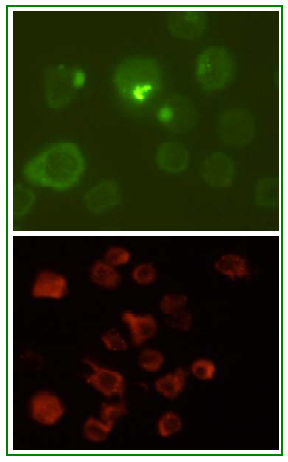
Figure 3. An example of fluorescence technique showing the receptor-ligand binding. Fluorescein (green) and rhodamine (red) labeled insulin was applied to the cultured cells which expresses insulin receptor on its surface. After certain incubation period and subsequent processing, cells were observed in fluorescent microscope. The rounded staining pattern shows fluorescein (green) and rhodamine (red) labeled insulin is bound to the insulin receptor on the cell membrane. (Images are captured in Olympus Fluorescence Inverted Microscope, magnification- 600) Principles of Excitation and Emission
The basic function of a fluorescence microscope is to irradiate the specimen with a desired and specific band of wavelengths, and then to separate the much weaker emitted fluorescence from the excitation light. The difference between the exciting and emitting wavelengths, known as Stokes shift is the critical property that makes fluorescence so powerful. By completely filtering out the exciting light without blocking the emitted fluorescence it is possible to see only the objects that are fluorescent. In a properly configured microscope, only the emission Fluorescence microscopy has become an ideal microscopy technique for the examination of all biological specimens, fixed or alive, because it allows the selective and specific detection of molecules at small concentrations to good signal to background ratio. The modern fluorescence microscope combines the power of high performance optical components with computerized control of the instrument and digital image acquisition to achieve a level of sophistication that far exceeds that of simple observation by the human eye. Microscopy now depends heavily on electronic imaging to rapidly acquire information at low light levels or at visually undetectable wavelengths.
In this post genomic era, the information of complete genome sequences and the identification and systematic cloning of human cDNAs are providing us with the challenging opportunity to analyze the complexity of biological processes on a large scale, with the goal of reaching a more complete description of their molecular regulation. For this purpose, many high-throughput techniques have been developed and successfully applied to diverse biological questions. However, despite their great usefulness, those techniques cannot provide adequate temporal or spatial resolution and, most importantly, they do not directly show whether the identified molecules have a functional role in the cellular process that is under investigation. |
|
The advantage of fluorescence for microscopy is that you can often attach fluorescent dye molecules to specific parts of your sample, so that only those parts are the ones seen in the microscope. You can also use more than one type of dye. By changing the excitation light, you can cause one type of dye to fluoresce, and then another, to distinguish two different parts of your sample.
How does a fluorescence microscope works?
In the figure 2 below, it is supposed that the excitation light needs to be violet, and the emitted light is red. The microscope uses a special dichroic mirror (or more properly, a "dichromatic mirror"). This mirror reflects light shorter than a certain wavelength, and passes light longer than that wavelength. Thus your eye only sees the emitted red light from the fluorescent dye, rather than seeing scattered purple light. The purple and red bars next to the dichroic mirror represent additional filters to help prevent the different wavelengths of light from going the wrong directions.
This particular style of fluorescence microscopy is known as epi-fluorescence, and uses the microscope objective to illuminate the sample (rather than illuminating the sample from the other side, which would be trans-fluorescence). 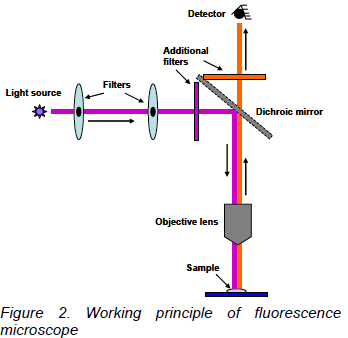
light should reach the eye or detector so that the resulting fluorescent structures are superimposed with high contrast against a very dark (or black) background. The limits of detection are generally governed by the darkness of the background, and the excitation light is typically several hundred thousand to a million times brighter than the emitted fluorescence. Fluorescence Detection
There are four essential elements of fluorescence detection systems: 1) an excitation source, 2) a fluorophore, 3) wavelength filters to isolate emission photons from excitation photons and 4) a detector that registers emission photons and produces a recordable output, usually as an electrical signal or a photographic image. Regardless of the application, compatibility of these four elements is essential for optimizing fluorescence detection. Fluorescence instruments are primarily of four types, each providing distinctly different information: Spectrofluorometers and microplate readers measure the average properties of bulk (µL to mL) samples. Fluorescence microscopes resolve fluorescence as a function of spatial coordinates in two or three dimensions for microscopic objects (less than ~0.1 mm diameter). Fluorescence scanners, including microarray readers, resolve fluorescence as a function of spatial coordinates in two dimensions for macroscopic objects such as electrophoresis gels, blots and chromatograms. Flow cytometers measure fluorescence per cell in a flowing stream, allowing subpopulations within a large sample to be identified and quantitated. Other types of instrumentation that use fluorescence detection include capillary electrophoresis apparatus, DNA sequencers and microfluidic devices. Each type of instrument produces different measurement artifacts and makes different demands on the fluorescent probe. For example, although photobleaching is often a significant problem in fluorescence microscopy, it is not a major impediment in flow cytometry or DNA sequencers because the dwell time of individual cells or DNA molecules in the excitation beam is short. Fluorescence-based imaging assays in intact living cells overcome these limitations because they can probe the function of macromolecules in their natural environment with exquisite and ever increasing spatial and temporal resolution.
The description of new imaging modalities such as confocal and two photon microscopy, the creation of new fluorescent molecules and the discovery and exploitation of fluorescent proteins have triggered revolution in fluorescent microscopy techniques. Understanding excitation of and emission by fluorophores, principle of fluorescence microscopes and ways of optimization of fluorescence process are now prerequisite for taking advantages of many of these developments.
About the Author: Mohammad Abul Farah attended Aligarh Muslim University in India where he received his M.Sc. and Ph.D. in Zoology with specialization in Genetics. He also served as Senior Research Fellow of Council of Scientific and Industrial Research, India. At present, he is working as a Research Scientist in Proteonik Inc., a biotechnology venture company based in Seoul, South Korea, on Diabetes research focusing on insulin signaling pathway. He can be reached at: farahkorea@yahoo.com |