Thermodynamic Properties and Alloying Behaviour of Liquid binary AlloyAshwani Kumar and S. M. Rafique
,1University Deptt. Of Physics, T.M.B.U., Bhagalpur-812007, Bihar, India e-mail: ashwani_kumar04@yahoo.co.in Abstract The complex formation model first proposed by Bhatia and Hargrove [1] assumes the existence of chemical complexes or pseudomolecules in liquid binary alloys. On the basis of this concept Singh and his coworkers [2-5] have studied various thermodynamical properties of different binary alloys. We have also studied different alloys within this framework [6-8]. The present paper envisages the study of some thermodynamic properties viz., Gibbs free energy of mixing (GM), Enthalpy of mixing (HM) and Entropy of mixing (SM) of the alloy under study. The results are in reasonable agreement with experiment and throw light on the ionic interactions of the constituent atoms leading to the alloying behaviour of the alloys under investigation. Keywords: Disordered Systems; Electronic Transport; Thermodynamic Properties. PACS 68.03-g, 64.70 Fx, 65.20+w, 61.25 Mv, 71.25 Cz. 1. Introduction A study of the recent literature reveals that in comparison to alkali metals, lesser amount of work has been done on liquid alkaline earth metals and their alloys [1]. The alloy Al-Mg has been acknowledged to be a good glass former [2]. The linear decrease in the melting temperature and flattened minimum in the intermediate region led Pearson [3] to believe that intermetallic compound Al3Mg2 exists in the solid state. He has also suggested Al12Mg17 while Samson and Gordon [4] have found Al30Mg23. In addition to its glass forming nature the alloy is useful in the production and design of low cost light metallic alloy [5]. Faber [6] has also confirmed the formation of chemical complexes. Recently we have carried out extensive and systematic studies on thermodynamic properties of Cu-Mg [7] and Ca-Mg [8] where the calculated data were compared with the available experimental results. In the present work, the thermodynamic study of Al-Mg is performed using the same formalism. The theoretical results have been compared with the experimental data 2. Thermodynamic Properties The thermodynamic properties viz., Gibb’s free energy of mixing (GM), the heat of formation (HM) and the entropy of mixing (SM) have been computed through the Bhatia-Hargrove technique [9]. The binary alloy is assumed to consist of a pseudo binary mixture of NA = Nc and NB = N(1-c) g moles of A and B atoms, and a type of AµB? of chemical complex. Here µ and ? are assumed to be small integers, c is the atomic fraction of A atoms and N is the Avogadro’s number. Following the complex formation model [9], we assume that the complex Al3Mg2 exist in the binary system. The formalism as already presented in our previous papers [7, 8] for other binary systems may be summarized as under 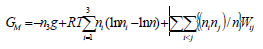 *Corresponding Author. Tel.: +91-641-2429840; 9973394775 (Mob.) E-mail address: ashwani_kumar04@yahoo.co.in (Ashwani Kumar) where g is the formation energy of the complex and it follows that the first term in the equation represents the lowering of the free energy due to the formation of complexes in the alloy, n3 is the number of complexes at equilibrium, Wij’s are the interaction energies and by definition they are independent of composition although they may depend on temperature and pressure, R is the molar gas constant and n is the total number of atoms in the case of compound formation. Once the expression for GM is obtained, other thermodynamic and microscopic functions, which are related to GM through standard thermodynamic relation, follow readily as, 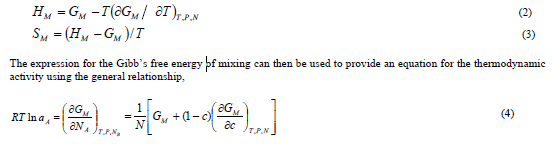 The first thing to do is to fit the values for the parameters g and Wij. In order to do this, we first calculate the value of g at the chemical concentration c = µ / (µ+?) using as a starting point, g ˜ - (µ + ? )GM. The energy parameters W12, W13 W23 are then adjusted in order to reproduce as closely as possible, the experimentally measured concentration dependence of the Gibb’s free energy of mixing. Once the energy parameters have been selected they remain the same for all mixing. (i). Wij and GM: The result of the above computations has been presented in Fig.1 for inspection alongwith the experimental values of GM from Hultgren et al. [10]. The interaction energies obtained through the fittings are W12= - 0.60, W13= 0.50, W23= - 0.50 and g = 1.50 at 1073 K (in terms of RT). On experimental grounds a smaller value of g < 3.5 suggests that Al-Mg is a weakly interacting system in the light of formation energy g and it comes in the category of the Mg-Sn, Ag-Al and Cu-Sn systems, in contrast to the strongly interacting systems like Hg-Na, Hg-K, Tl-Te, Mg-Bi etc. for which the formation energies are much larger i.e., 8.294, 9.965, 10.84, 16.7 respectively. Further, we observe that the interaction energies W12 and W23 are attractive while W13 is repulsive in nature. This also supports the weakly interacting nature of Al-Mg alloy. The free energy of mixing GM shows a good agreement with experiment (Fig.1) and GM = - 0.8890 at the equiatomic composition also speaks of a weakly interacting system. Also we observe that GM is symmetrical for Al-Mg while it is found to be asymmetric for alloys like Al-Ca, Ca-Mg, Mg-Zn and Cu-Mg etc. However n3 is not maximum at the equiatomic composition cMg = 0.5 rather it is maximum near cMg = 0.4 which indicates the formation of complex Al3Mg2. (ii). HM and SM : A perusal of Fig.2 showing the heat of formation HM and entropy of mixing SM of Al-Mg alloy for various concentrations at 1073 K alongwith the experimental data of Hultgren et al. [10] brings out the fact that although the heat of mixing HM is in fair agreement with experiment the entropy of mixing from cMg = 0.3 to 0.6 shows a departure predicting smaller values than the experimental ones. 3. Structure Factors The experimental data of partial and total structure factors of binary alloys are not available at different compositions; also they have insufficient accuracy [11]. Thus one has to divulge into computational effort and this is achieved through the hard sphere reference system, obtaining the solutions of Percus-Yevik equation for mcomponent hard sphere mixture (Hiroike [12] and Hoshino [13]). The total structure factor S(k) is given by  where, Sij(k) are the partial structure factors, c1 = n1/n, c2 = n2/n, c3 = n3/n are the concentration fractions of the scattering centers A, B and AµB? (the chemical complex). Here q is the wave vector and c1, c2, c3 being the concentration fractions of the scattering centers A, B and AµB? (A= Ca, B= Mg, µ = 1, ? = 2). These computations need two ingredients viz., hard sphere diameters si and packing fraction ? related by 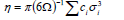 ci are the concentrations of the species and O is the volume of the alloy. The hard sphere diameters s1and s2 are calculated by matching the first peak of the structure factors of the constituent elements at their melting temperature. They have been assumed to be independent of temperature and concentration and s3 has been taken to be 0.11 nm for Al-Mg alloy. The number of complexes n3 along with the above mentioned hard sphere diameters are used to compute the partial and total structure factors. The computed partial structure factors and the total structure factor at c = 0.4 and 0.5 have been shown in Fig.3 against ? = k / kF, kF being the Fermi wave vector. A perusal of Fig.3 bring out the following facts (i). S33(k) remains positive throughout the range of ? = k / kF. (ii). S11(k) and S22(k) are negligibly negative at ? = 0.4 only and then they become positive. (iii). S12(k), S13(k) and S23(k) have both positive and negative values like S11(k) and S22(k). (iv). At equiatomic composition, S22(k) is higher than S11(k) and S33(k). It should be mentioned that, the total structure factor S(k) shows the behaviour of random mixing without a sub-peak or asymmetry of the first peak and shows the behavior of compound forming with a sub-peak below the first peak [14]. The partial structure factors of unlike atom pairs have maxima, which lies in between those of like pair of atoms in case of random mixing. On the other hand, in compound forming alloys the partial structure factor of unlike atom pairs have a very sharp peak with a sub-peak below the main peak. Various alloys also show their behavior in between these two types. Negative peak in the lower region of k / kF indicates preference for unlike nearest neighbors. The humps in the lower k regions are due to short-range order (SRO) with preference for unlike nearest neighbors [15]. Positive values of partial structure factors imply that the repulsive core part of the effective interionic potential is dominating whereas the negative values imply that the dominating part is attractive in nature. 4. Concentration-Concentration Fluctuation, SCC(0) The stability and microscopic structures of binary alloys may be studied through the evaluation of Bhatia-Thornton partial structure factors for the long wavelength limit q ? 0. This has been termed as concentration-concentration structure factors SCC(q) related to Ashcroft-Langreth structure factors Sij(q) as 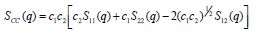 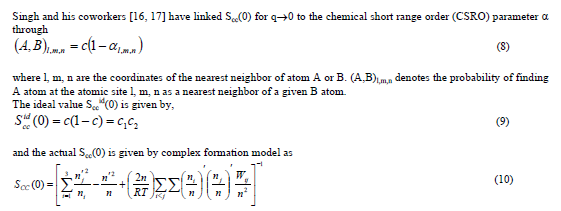 where a prime denotes differentiation with respect to c1. On the other hand, Scc (0) computed through the experimental Gibb’s free energy of mixing are termed as experimental Scc (0), obtained through 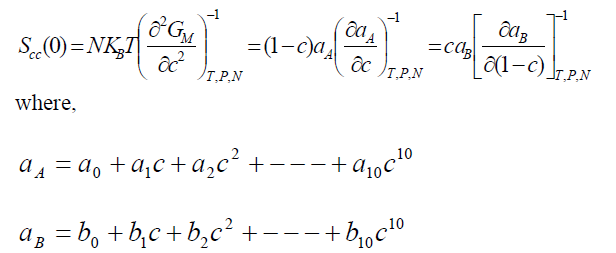 At temperature above Debye temperature and in the long wavelength limit Scc(0) represents the mean square thermal fluctuations in the particle. The long wavelength limit (Scc(0)) of the concentration-concentration structure factor [18] is of considerable importance [19, 20] to study the nature of atomic order in binary liquid alloys. The basic advantage of Scc(0) is that one can determine it making use of the thermodynamic relations. The last two equalities of Eqn. (11) can be used directly to compute Scc(0) from observed numerical data. This is usually known as experimental values of Scc(0). It is possible to use the variation of Scc(0) with concentration to understand the nature of atomic order in liquid alloys. Basically, the deviation of this quantity from its ideal values given by Scc id(0) = c1c2 is significant in explaining the interaction between the components of the binary mixture. The basic inference is that Scc(0) < Scc id(0) implies a tendency for heterocoordination (preference of unlike atoms to pair as nearest neighbours), while Scc(0) > Scc id(0) implies homocoordination (preference of like atoms as nearest neighbours). For a demixing system, Scc(0) >> Scc id(0). The experimental determination of Scc(0) from diffraction experiments is quite complicated, however, it can be calculated from the measured activity data. 5. Chemical Short Range Order (CSRO) parameter The chemical short range order parameter (CSRO) is yet another important parameter proposed by Warren [21] and Cowley [22]. In order to measure the degree of order in the liquid alloy, the Warren – Cowley short-range order parameter [23, 24] a can be computed. Experimentally, a can be determined from the knowledge of the concentration-concentration structure factor SCC(q) and the number-number structure factors SNN(q). However, in most diffraction experiments, these quantities are not measurable. On the other hand, a can be estimated from the knowledge of SCC(0) [25]. Knowledge of a provides an immediate insight into the nature of the local arrangement of atoms in the mixture. a = 0 corresponds to a random distribution, a < 0 refers to unlike atoms pairing as nearest neighbors whereas a > 0 corresponds to like atoms pairing in the first coordination shell. From a simple probabilistic approach, one can show that the limiting values of a lie in the range 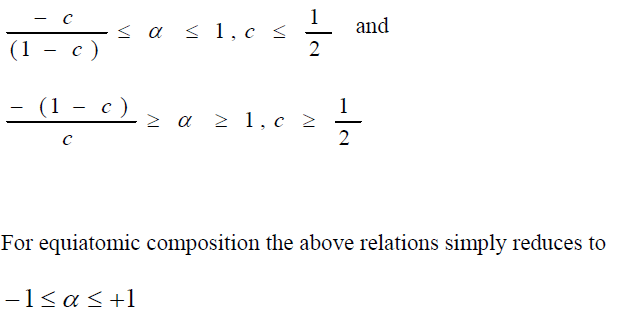 The minimum possible value of a is amin = - 1, which implies complete ordering of unlike atoms as nearest neighbors. On the other hand, the maximum value amax = +1 implies total segregation leading to phase separation. The computed CSRO is presented in Fig. 4 for perusal. From Fig.4 we observe that for Al-Mg we get Scc(0) < Scc id(0) for all concentrations. Al-Mg is found to be a glassy alloy both towards Al and Mg rich end while a is throughout negative. The theoretical and experimental data of Scc(0) are in good agreement. 6. Surface Tension From the point of view of theoretical modeling of surface properties, a statistical mechanical approach, which is derived from the concept of a layered structure [26] near the interface, is very useful. This has been used with great success to model the surface tension in binary liquid alloys [27-29]. The grand partition functions set up for the surface layer and that of the bulk [30] provide a relation between surface (ci s) and bulk (ci) compositions. The resulting expressions are: 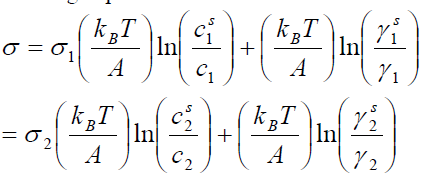 where si (i = 1,2) are the surface tensions of the pure components at specified temperature, ?i = ai / ci and ?i s = ai / ci s are the activity coefficients, while ci s and ?i s (i = 1,2) are the component at the surface, respectively. The mean atomic surface area Ai can be calculated from the relation: 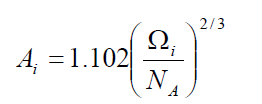 where NA is the Avogadro’s number and Oi is the atomic volume. The mean surface area A of the alloy has been calculated from the relation A = S ci Ai. In conformity with the concept of layered atomic structure near the interface, ?i and ?i s are assumed to be related as: 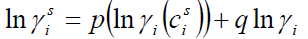 sm being the surface tension at melting temperature. The computed surface tensions at different concentrations have been shown in Fig.5 for perusal. It shows that the surface tension of Al falls sharply with the addition of Mg component. For pure Al, it is 865.0 while with the addition of Mg component it becomes 581.1mNm-1 at (c = 0.2). Afterwards the surface tension goes on decreasing to 501.5 at cMg =0.9. At the Mg end, it slightly increases to 506.8. 7. Electrical Resistivity The liquid electrical resistivity of binary alloys is computed through the well-known Faber-Ziman formalism. The form factor w (k, q) have been obtained through Heine – Abarenkov model potential represented by 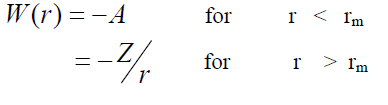 and the local unscreened form factor is given by  The concentration dependence of computed electrical resistivity of Al-Mg has been shown in Fig.6. Fig.6, shows that the liquid electrical resistivity of Al-Mg ranges from 13.49 µ O cm (cMg =0) to 38.19 µ O cm at equiatomic composition, finally decreasing to 14.16 (cMg =1). The electrical resistivity of this system is found to be symmetric about cMg =0.5 (equiatomic composition). No anomalous value is obtained at any concentration. 9. Conclusions The present study based on the Percus-Yevik hard sphere reference system, complex formation model of binary alloys has been successfully applied to the study of the alloying behaviour of Al-Mg alloy at 1073 K. An insight into the structural and compositional details has been obtained through this study. References [1]. C. E. Gonzalez, D. J. Gonzalez, J. M. Lopez, J. Phys. Cond. Matt., 13 (2001) 7801. [2]. T. R. Ananta Raman, Metallic Glasses: Properties and Application (Ed. P. R. Rao), 1984. [3]. W. B. Pearson, A Handbook of Lattice Spacing and Structure of Binary Alloys (Metals Park, Ohio, ASM), 1967. [4]. S. Samson, E. K. Gordon, Acta Crys., 24 (1968) 1004. [5]. R. Agrawal, S. G. Fries, H. L. Lukas, G. Petzon, F. Sommer, T. G. Chan, E. Effenburg, Z. Metallk., 83 (1992) 216. [6]. T. E. Faber, Introduction to the Theory of Liquid Metals (Camb. Oxford Press, London), 1972. [7]. Ashwani Kumar, S. M. Rafique, N. Jha, A. K. Mishra, Physica B, 357 (2005) 445. [8]. Ashwani Kumar, S. M. Rafique, N. Jha, Physica B, 373 (2006) 169. [9]. A. B. Bhatia, W. H. Hargrove, Phys. Rev. B, 10 (1974) 3186. [10]. R. Hultgren, P. D. Desai, D. T. Hawkins, M. Gleiser & K. K. Kelley, Selected Values of the Thermodynamic Properties of Binary Alloys, ASM, Materials Park, OH, 1973. [11]. Y. Waseda, The structure of non-crystalline materials, Liquid and Amorphous Solids, Mc. Graw Hill, N.Y., 1980. [12]. K. Hiroike, J. Phys. Soc. (Japan), 27 (1969) 1415. [13]. K. Hoshino, J. Phys. F, 13 (1983) 1981. [14] Y. Waseda, Inst. Of Phys. Conf. Series, No. 30, pp. 230, 1970. [15] H. Reiter, H. Ruppersberg, W. Speicher, Inst. Of Phys. Conf. Series, No. 30, pp.133, 1977. [16] R. N. Singh, N. H. March, Intermetallics Compounds: Principles and Practices (Ed. J. H. Westbrook and R. L. Fleischer), John Wiley and Sons, N.Y., 1995. [17] S. M. Osman, R. N. Singh, Phys. Rev. E, 51 (1995) 332. [18] A. B. Bhatia, D. E. Thornton, Phys. Rev. B, 2 (1970) 3004. [19] P. Chieux, H. Ruppersberg, J. Phys. Coll. C, 8 (1980) 41. [20] C. N. J. Wagner, Rapidly Quenched Metals, (Ed. S. Steeb, H. Warlemount), North-Holland, Amsterdam, pp.405, 1985. [21]. B. E. Warren, X-ray diffraction, Addison-Wesley, Reading in M.A., 1969 . [22]. J. M. Cowley, Phys. Rev., 77 (1950) 667. [23] A. B. Bhatia, R. N. Singh, Phys. Chem. Liq., 13 (1984) 177. [24] R. N. Singh, Can. J. Phys. 65 (1987) 309. [25] R. N. Singh, D. K. Pandey, S. Sinha, N. R. Mitra, P. L. Srivastava, Physica B, 145 (1987) 358. [26] E. A. Guggenheim, Mixtures, Oxford University, Oxford, 1952. [27] L. C. Prasad, R. N. Singh, V. N. Singh, G. P. Singh, J. Phys. Chem., B102 (1998) 921. [28] B. C. Anusionwu, O. Akinlade, L. A. Hussain, J. Alloys Comp., 278 (1998) 175. [29] L. C. Prasad, R. N. Singh, Phys. Rev. B, 44 (1991) 13768. [30] L. C. Prasad, R. N. Singh, G. P. Singh, J. Phys. Chem. Liq., 27 (1994) 179. 49 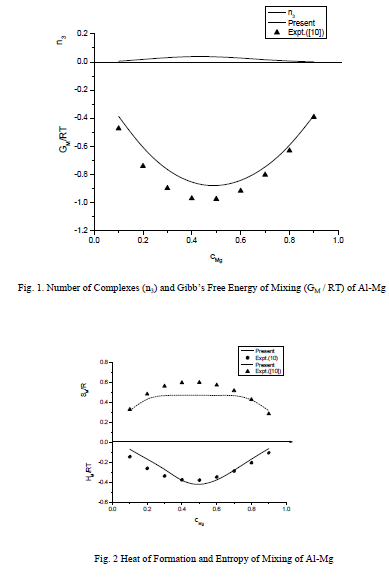 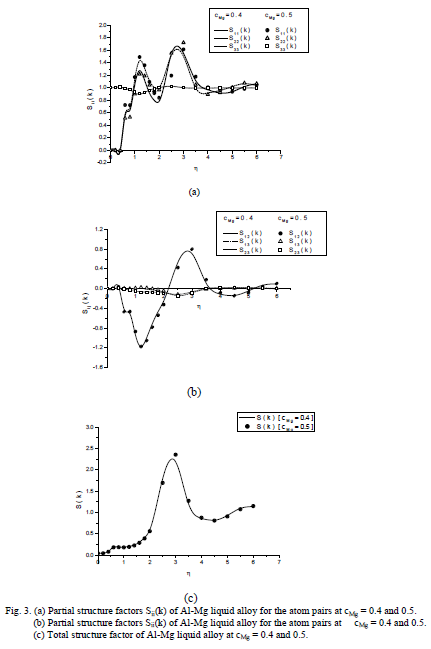 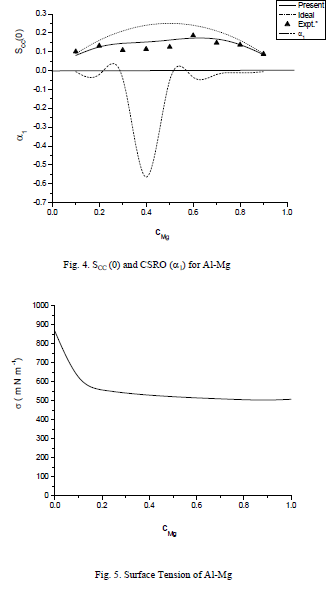 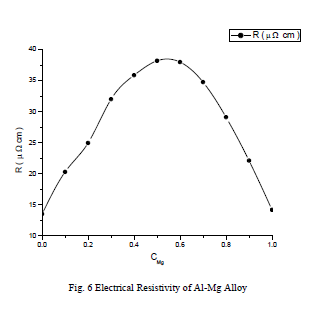
|
|

